| contact us |
| why choose Chiralabs? |
Circular Dichroism Applications:
Stereochemical Assignment
One of the primary roles of CD is in the stereochemical assignment of a chiral molecule, either in relative or absolute terms. Currently, the ability to calculate the electronic CD a priori is limited and thus the use of CD is typically through comparison with previously acquired spectra of compounds of known stereochemistry that have either the same or closely analogous molecular structures to that under investigation. One exception to this is for molecules containing two or more similar highly absorbing chromophores, for which exciton coupling theory may be applied to obtain a definitive absolute stereochemistry.A related stereochemical assignment technique is the use of molecular tweezers that exhibit an induced CD via an exciton coupling mechanism, as described in the CD application note of on Interactions.
“Quadrant Rules” were popular in the early days of CD spectroscopy, being geometric arguments to “predict” the sign of CD bands. However, over a long period evidence has accumulated of many compounds that break the predicted patterns, such that the rules have lapsed into ill-repute. Nonetheless, in the right circumstances, they can be a valuable tool to aid interpretation [Macleod et al 2004, Butz et al 2002].
Alternatively, the CD spectrum provides a definitive, signed, reference for relative stereochemical assignment. With certain caveats, series of compounds can be compared as to whether they are of corresponding or opposite hand to each other. Thence, once the absolute stereochemistry of one compound is established, perhaps through crystallographic studies, the absolute handedness of all of the series are likewise known.
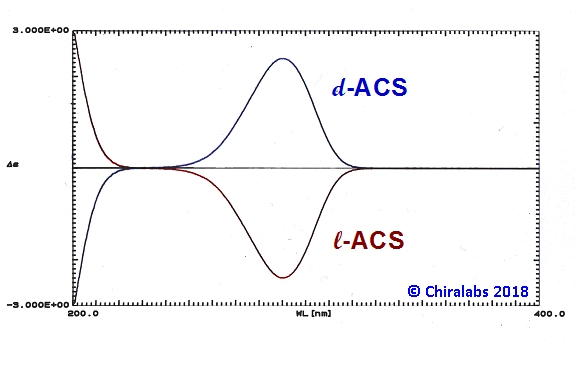
The figure below depicts the CD spectra of the l-(1R,4S)-(-) and d-(1S,4R)-(+) enantiomers of ammonium 10 camphor sulphonate in the UV wavelength region. It is immediately apparent that the two spectra are “mirror-images” of each other, consistent with the two compounds being enantiomeric. The signs of the peaks in the CD spectrum provide a definitive indication of the handedness of the compound. In this case the d-enantiomer has a positive CD at 290.5nm, with a negative peak at shorter wavelength. Oppositely signed CD is seen for the opposite enantiomer.
- please contact us to see how we may enhance your analytical capabilities or help solve your analysis problems.
References
Macleod, NA, Butz, P, Simons, JP, Grant, GH, Baker, CM, Tranter, GE,
Electronic circular dichroism spectroscopy of 1-(R)-phenylethanol: The "Sector rule" revisited and an exploration
of solvent effects, Isr J Chem, 2004, 44, 27 – 36.
Butz P, Tranter GE, Simons JP, Molecular conformation in the gas phase and in solution, Phys Chem Comm, 2002, 5, 91 – 93.